First of all, a quick introduction to 4 the most important figures in thermodynamics:
- Sadi Carnot (1796-1832) who discovered the second law of thermodynamics.
- Rudolf Clausius (1822-1888) who restated the Sadi Carnot’s principle into the form we use today.
- William Thomson (Lord Kelvin, 1824-1907) who brought thermodynamics into its modern form.
- Max Planck (1858-1947) who is best known for the Planck Function of radiation, heat transfer and as a founder of quantum mechanics.
Essentially all thermodynamic system problems can be cast in a similar form: an isolated system, divided into 2 subsystems by a piston. The question arises when what happen when the piston is:
- adiabatic, fixed, and impermeable
- diathermal (heat transfer is allowed)
- allowed to move
- porous to one or more chemical species
Postulatory Basis
The postulates should be the simplest ones that are proved a posteriori and will do the job, rather than be derived by an a priori proof.
| a priori | deduction, based on theory (Theory → Hypothesis → Observation → Confirmation) |
| a posteriori | induction, based on experiment (Observation → Pattern → Tentative Hypothesis → Theory) |
| ab initio | from the beginning |
| ipso facto | by the fact |
Postulate 1
There exists certain states (equilibrium states) of simple systems that, macroscopically, are characterized completely by the internal energy U, the volume V, and the more numbers N1, N2, …, Nr, of the chemical components, where r is the number of chemical components.
The implications of this postulate:
- Previous history plays no role in determining the final state. When systems are in equilibrium, the property functions do not depend on any dynamic quantities.
- All microscopic quantum states are allowed (a priori probability).
- There are violations of this postulate: heat treated steel, non-Newtonian fluid with hysteresis, ortho- and para- hydrogen.
Postulate 2
There exists a function called the entropy S, of the extensive parameters of any composite system, defined for all equilibrium states and having the property that: the values assumed by the extensive parameters are those that maximize the entropy over the manifold of constrained equilibrium states.
This postulate introduces the concept of entropy and essentially states the second law of thermodynamics.
| Extensive properties | Those directly depend on the size (total extent) of the system. For example: internal energy U, total volume V, total mass M or moles N, total entropy S, enthalpy H, etc. |
| Intensive properties | Normalized or not dependent on system size. For example: temperature T, pressure p, chemical potential μ, etc. |
| Normalized properties | Extensive properties normalized on mass, moles or volume. For example: internal energy u, enthalpy h, entropy s. |
Postulate 2 only applies for equilibrium states. Also the total entropy (including all subsystems and surrounding) is maximized. We can solve the Fundamental Problem if we know the Fundamental Relation:
S = S(U, V, Ni)Examples of Fundamental Relations include Van der Waal’s Liquid, Ideal Gas, etc.
Postulate 3
The entropy of a composite system is additive over the constituent subsystems. The entropy is continuous and differentiable and is a monotonically increasing function of energy.
This postulate simplifies the mathematics: the entropy of the overall system is the sum of the entropies of each subsystem, i.e. S = ∑j Sj. Next, entropy of each subsystem is a function of the properties of that subsystem only Sj = Sj(Uj, Vj, Ni,j). Third, entropy is a 1st-order function of the extensive properties, i.e. S(λU, λV, λNi) = λS(U, V, Ni).
The partial derivative of entropy is always positive ∂S/∂U > 0, which means entropy can be inverted with respect to energy in the fundamental equation, U = U(S, V, Ni). Lastly, the extensive properties can be normalized: u ≡ U/N, v ≡ V/N, s ≡ S/N, further more we have
S = N S(U/N, V/N, Ni/N)
s = s(u, v, yi) # yi is the mole fractionPostulate 4 (3rd Law of Thermodynamics)
The entropy of any system vanishes in the state for which
∂S/∂U = 0.
This implies entropy goes to zero as temperature goes to zero.
Temperature
Consider an isolated system composed of 2 subsystems A and B which are initially at different equilibrium states. At the beginning, the barrier between the 2 subsystems was fixed, insulated and impermeable, and nothing could happen. ONLY heat transfer is then allowed across the barrier. If there is a difference in temperature, the heat transfer will occur. But how much?
The way to answer is to find the final equilibrium state, and use the first law to calculate the heat transfer that took place between the initial and final states. The postulates give the rule for finding an equilibrium state. Assuming there is a mixture of species, taking the differential of S gives us:
dS = ∂S/∂U dU + ∂S/∂V dV + ∑j ∂S/∂Nj dNjIf the volume V and composition Nj are fixed, then only the first partial derivative will have a value. The total entropy S = SA + SB. Differential of S now is:
dS = dSA + dSB
⟹ dS = ∂SA/∂UA dUA + ∂SB/∂UB dUBNow note the energy within the overall system must be conserved U = UA + UB, so that dU = dUA + dUB = 0, and –dUA = dUB. Then eliminate dUB in dS and set dS to zero.
dS = [∂SA/∂UA - ∂SB/∂UB] dUA = 0
⟹ [∂SA/∂UA - ∂SB/∂UB] = 0
⟹ ∂SA/∂UA = ∂SB/∂UB
⟹ ∂UA/∂SA = ∂UB/∂SB ≡ T
⟹ TA = TBThe standard thermodynamic definition of temperature T is the partial derivative of internal energy U with respect to entropy S, i.e. T = ∂U/∂S.
Pressure
Consider an isolated system composed of 2 subsystems A and B which are initially at different equilibrium states. At the beginning, the barrier between the 2 subsystems was fixed, insulated and impermeable, and nothing could happen.
Both heat transfer across the barrier and motion of barrier are then allowed. If without heat transfer, there is no mechanism to stop the barrier (piston) from oscillating back and forth forever. It is the friction that slows down the piston at its equilibrium position. Friction generates heat, and thus heat transfer.
So now only Ni is fixed, and U and V change. The differential form of Fundamental Relation is:
dS = dSA + dSB
⟹ dS = ∂SA/∂UA dUA + ∂SA/∂VA dVA + ∂SB/∂UB dUB + ∂SB/∂VB dVBNow note the total volume is fixed V = VA + VB, so that dV = dVA + dVB = 0, and –dVA = dVB. Then together with temperature, we eliminate dUB and dVB in dS and set dS to zero.
dS = [∂SA/∂UA - ∂SB/∂UB] dUA + [∂SA/∂VA - ∂SB/∂VB] dVA = 0
⟹ [∂SA/∂UA - ∂SB/∂UB] = 0 and [∂SA/∂VA - ∂SB/∂VB] = 0
⟹ ∂SA/∂UA = ∂SB/∂UB and ∂SA/∂VA = ∂SB/∂VB
⟹ ∂UA/∂SA = ∂UB/∂SB ≡ T and ∂SA/∂VA = ∂SB/∂VB ≡ p/T
⟹ TA = TB and pA = pBThe standard thermodynamic definition of pressure p is temperature T times the partial derivative of entropy S with respect to volume V, i.e. p = T ∙ ∂S/∂V.
Chemical Potential
Chemical potential plays exactly the same role for mass transfer, as temperature and pressure do for heat transfer and volume change or work.
Consider an isolated system composed of 2 subsystems A and B which are initially at different equilibrium states. At the beginning, the barrier between the 2 subsystems was fixed, insulated and impermeable, and nothing could happen. Both heat transfer and mass transfer across the barrier are allowed.
Assume we have only one species, so i = 1. Now only N1 and U change. The differential form of Fundamental Relation is:
dS = dSA + dSB
⟹ dS = ∂SA/∂UA dUA + ∂SA/∂N1,A dN1,A + ∂SB/∂UB dUB + ∂SB/∂N1,B dN1,BNow note the total mass is fixed N1 = N1,A + N1,B, so that dN1 = dN1,A + dN1,B = 0, and –dN1,A = dN1,B. Then together with temperature, we eliminate dUB and dN1,B in dS and set dS to zero.
dS = [∂SA/∂UA - ∂SB/∂UB] dUA + [∂SA/∂N1,A - ∂SB/∂N1,B] dN1,A = 0
⟹ [∂SA/∂UA - ∂SB/∂UB] = 0 and [∂SA/∂N1,A - ∂SB/∂N1,B] = 0
⟹ ∂SA/∂UA = ∂SB/∂UB and ∂SA/∂N1,A = ∂SB/∂N1,B
⟹ ∂UA/∂SA = ∂UB/∂SB ≡ T and ∂SA/∂N1,A = ∂SB/∂N1,B ≡ -μ/T
⟹ TA = TB and μ1,A = μ1,Bμ is called chemical potential. Note the minus sign, i.e. μ = -T ∙ ∂S/∂N, because we want the mass transfer to go from the region of higher chemical potential to lower chemical potential.
This concept applies to chemically reacting mixture as well. For a fixed volume V and energy U, this is one equations with r unknowns, if there are r species:
dS = ∑ri=1 μi/T dNi = 0If we take atom conservation into account, then it turns out there are enough equations for the number of unknowns as long as we know the initial condition.
pk = ∑ri=1 nki NiRepresentations
By representation, we mean the division of properties: the relations between dependent and independent. For example, in the case of the simple compressible substance, only 2 independent properties are needed to determine all the other properties, say U = U(p, V), or U = U(S, p). Each choice of the two independent properties is a different representation.
We have introduced the Fundamental Relation S = S(U, V, Ni), and its differential form is called Entropy Representation:
dS = 1/T dU + p/T dV - ∑ri=1 μi/T dNiHowever because of the Postulate 3, we can also write U = U(S, V, Ni), and its differential form is called Energy Representation:
dU = T dS - p dV + ∑ri=1 μi dNiEntropy representation and energy representation contain the same information.
There are as many representation as there are possible combinations of independent variables. The problem is how to retain all the information in the Fundamental Relation when transforming between independent and dependent properties.
Equations of State
In both of the entropy representation and energy representation, the coefficients of the differential form are intensive parameters (T, p, μ) which can be calculated as a function of the independent properties (S, V, Ni) given the Fundamental Relation, i.e.:
T = T(S, V, Ni)
p = p(S, V, Ni)
μ = μ(S, V, Ni)These relationships are formally called Equations of State. If the Equations of State are known, they can be integrated to recover the Fundamental Relation. This is true in any representation.
Euler Equation
From the definition of the homogenous first-order property, the Fundamental Relation could be written in a convenient form: U(λS, λV, λN) = λU(S, V, N), which means multiplying the independent variable S, V, N with a constant λ, results in the dependent variable U being multiplied by the same factor.
Differentiating both side of the equation with regard to λ, we obtain this expression:
∂U/∂(λS) S + ∂U/∂(λV) V + ∂U/∂(λN) N = U(S, V, N)Select λ = 1, we got Euler Equation:
U = T S - p V + ∑ri=1 μi Ni
⟹ S = 1/T U + p/T V - ∑ri=1 μi/T NiEuler Equation provides explicit relationship between the intensive and extensive properties.
Gibbs-Duhem Relation
If we differentiate the Euler Equation and using the differential form of the Fundamental Relation, we derive the Gibbs-Duhem Relation, which shows not all the intensive properties are independent of each other:
S dT - V dp + ∑ri=1 Ni dμi = 0The actual number of independent intensive parameters is called the Thermodynamic Degrees of Freedom. A simple system of r components has r+1 degrees of freedom. This corresponds directly to Gibbs Phase Rule.
Alternative Representations
It is often useful to have one or more of the intensive properties as independent. The Legendre transform allows transforming between extensive and intensive properties so that the transform relation contains all the information in the Fundamental Relation.
Various transforms from the energy representation are called Thermodynamic Potentials, because differences in their value can drive action.
Various transforms from the entropy representation are called Massieu Functions.
Extreme Principle: entropy and its transformation are maximized; and energy and its transformation are minimized.
Quasi-Static Process
A quasi-static process is one that occurs slowly enough so that the system appears to be in equilibrium at all times. The practical consequence is that the Fundamental Relation applies during the process. This is an idealization that will be very useful in calculating limiting behavior. It leads to the concept of reservoirs.
Reservoirs are useful idealizations for the way systems interact with their surroundings. We usually make some idealized assumptions about them that leads to simplification. Two commonly used are:
| mechanical energy reservoir | Idealization of a very large mechanical sink or source (a giant piston cylinder arrangement). The properties of the reservoir are unchanged even through work is done on / by it. Consider an infinitesimal amount of work dW, then MER is isentropic. dU = dW = -pdV ⟹ 1/T dU + p/T dV = 0 = dS |
| thermal energy reservoir | Idealization of a very large volume whose properties are essentially unaffected when heat is added or subtracted. Consider an infinitesimal amount of heat transfer dQ, then TER is NOT isentropic. dU = dQ ⟹ 1/T dU = 1/T dQ = dS |
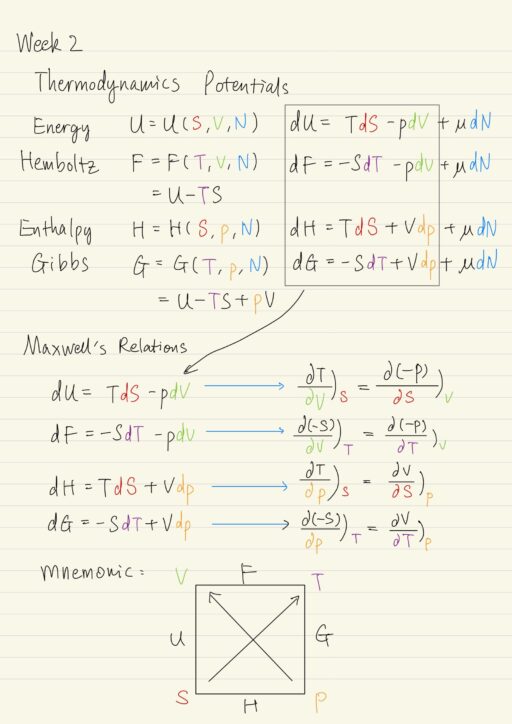
Maxwell’s Relations
James Clerk Maxwell was a 19th century mathematical physicist. Besides Maxwell’s equations of classical electromagnetism, Maxwell also worked on thermodynamics. If we start with the differential form of various thermodynamic potentials, it leads to Maxwell’s Relations.
Building Property Relations
We seek the properties as a function of various independent variables. If we know the Fundamental Relation, then we can use the Legendre transforms and intensive properties definitions to obtain any relationships we might need. Alternatively, we often have experimental data or theoretical results in a particular functional form but need properties in a different form. Traditionally, the experimental approach was to measure the p-V-T relationship and the specific heat.
My Certificate
For more on Macroscopic Thermodynamics, please refer to the wonderful course here https://www.coursera.org/learn/macroscopic-microscopic-thermodynamics
Related Quick Recap
I am Kesler Zhu, thank you for visiting my website. Check out more course reviews at https://KZHU.ai