Thermodynamics is the study of the equilibrium behavior of systems, for which motion at the microscopic level of atom and molecules is important. It involves solving problems that arise because of work, heat transfer, mass transfer interactions with a working substance of some kind.
Microscopic behavior determines macroscopic properties such as PVT relations, internal energy and enthalpy, and entropy. What we see at the macroscopic level are averages of the microscopic level behavior.
| Macroscopic / Classical Thermodynamics | Assume the availability of thermodynamic property relations. |
| Microscopic / Statistical Thermodynamics | Tell how to calculate thermodynamic property relations. Quantum mechanics determines atomic and molecular structure. |
Microscopically, if a system is constantly undergoing changes and yet the average, the variance properties of the system remain unchanged, the system is in a stationary state, and said to be in equilibrium.
Systems, for which there are local spatial or time variations in macroscopically observable properties, are not in equilibrium.
The nature of matter
Matter is composed of atoms and molecules.
Atoms
A typical atom is 3-5 angstrom (1 A = 10-10 meters). Atoms are composed of positively charged nuclei and negatively changed electrons. The nuclei is further composed of positively charged protons and neutral neutrons.
Charge of protons and electrons is ±1.602 × 10-19 C. 1 Coulomb is charge carried in 1 sec by 1 Amp. Therefore, neutral atom must have the same number of protons and electrons. It is common to express the mass of atom in Atomic Mass Units (AMU), which is 1.66 × 10-27 kg (1/12 of the mass of the carbon 12 nucleus).
| mass of proton | 1.67 × 10-27 kg |
| mass of neutron | 1.67 × 10-27 kg |
| mass of electron | 9.109 × 10-27 kg (1/1833 of mass of proton) |
Atoms are held together by electronstatic forces. The force between 2 charges is given by Coulomb’s Law, where the permittivity of vacuum ε0 = 8.854 × 10-12 C2/Nm2.
F = 1/4πε0 q1q2/r2A typical atomic force is 10-9 N.
Molecules
Molecules are groups of atoms held together by electronstatic forces. There are two types of bonds: ionic and covalent.
| Ionic bonds | Typically formed between metals and non-metals, each of which has opposite charge. |
| Covalent bonds | Formed due to overlap between the electron fields of atoms. |
Molecules can contain anywhere from 2 to hundreds of thousands of atoms.
How can we predict macroscopic behavior from the microscopic behavior? There are 3 types of motion: vibration, rotation and translation. We usually can not solve equations of motions for all atoms and molecules. We will have to use the method of statistical math.
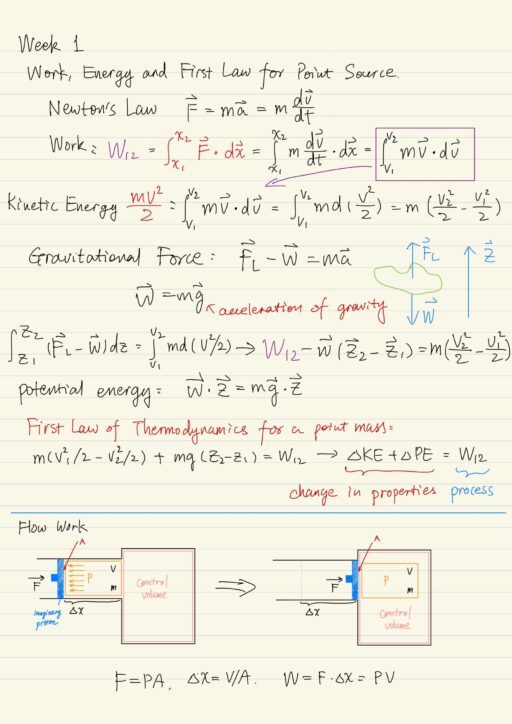
Work, Energy and the First Law for a Point Source
Work involves a force action through a distance: ∫F(x)∙dx. Also to perform the integral, the applied force has to be a function of the distance. Thus the work is a process.
We call mv2/2 the Kinetic Energy, it represents the state of the point mass at a given point in time and space. Thus it is a property of the point mass.
Gravity is important in many thermodynamic fluid dynamic problems as it adds an effective weight (a force) to systems understudy. We identify m∙g∙z as the potential energy. d(m∙g∙z) = m∙g∙dz is an exact differential, and thus potential energy is a property.
Putting all these together, we write the conservation of energy: the first law for a point mass.
m(v22/2 - v21/2) + mg(z2 - z1) = W12
→ ∆KE + ∆PE = W12| ∆KE + ∆PE | Properties describe the state of a system at a specific time and space. The state of a system is described by the values of its properties. E.g.: system mass, density, internal energy, entropy, pressure, temperature, kinetic energy, potential energy. |
| W12 | Processes describe those actions that result in a change of properties. E.g.: work, heat transfer, mass transfer. |
Internal Energy, Heat Transfer and the 1st Law for Real Systems
Open systems (control masses) and closed systems (control volumes) are no longer point masses. The main difference between the two is:
| Closed systems | Mass is fixed, no mass can cross the system boundaries. |
| Open systems | Mass can cross the system boundaries. |
First law will have additional terms if mass is allowed to cross the system boundary because mass can carry energy. Macroscopically we have kinetic energy and potential energy of the entire system. Microscopically, we have the energy of microscopic motion of the atoms and molecules that make up the system:
| Translation | kinetic energy of moving atoms / molecules. |
| Rotation | angular momentum of molecules. |
| Vibration | relative motion of bonded atoms in a force field |
Finally, orbiting electrons are in continual motion in a force field. We call the energy due to these internal modes of motion Internal Energy (U), thus at a macroscopic level:
Total Energy = Kinetic Energy + Potential Energy + Internal Energy
E = KE + PE + UThe kinetic energy due to internal, microscopic motion can be of the same order of magnitude as the macroscopic kinetic and potential energy. If we can access the internal energy either to increase or decrease it, there are endless possibility for putting it to work in useful ways.
Closed Systems
For closed systems, there are 2 ways to access internal energy: work and heat transfer. Both of them are processes and have dimensions of force times distances. Units are Joules.
- Work (W12) is organized (coherent) work, involving force acting through a distance.
- Conductive or convective heat transfer (Q12) is random, unorganized (incoherent) work, involving force acting through a distance at the microscopic level.
- Molecules in two adjacent media are striking each other randomly, such that there is a net transfer of energy.
- In radiative heat transfer, photons are absorbed or emitted by system mass at the molecular level.
The first law for a closed system:
∆KE + ∆PE + ∆U = W12 + Q12The change in total energy of the closed system must equal to the net work and the heat transfer acting on the system.
Open Systems
For an open systems, we must take into account the effect of mass flow into / out of the system. This is because mass carries energy per unit mass (u + V2/2 + gz). It also takes Flow Work to push the mass in or out of the system.
The force F is equal to the pressure difference P across the volume, times the cross-sectional flow area A. F = P A.
The distance x is equal to the volume V of material entering divided by the cross-sectional flow area A. x = V/A.
So the flow work is then W = P V. We more commonly express the flow work on a per unit mass basis, so that it becomes the pressure times the specific volume, or equivalently the pressure divided by the density ρ.
Wunit_mass = W / mass = P * Vunit_mass = P / ρA new property enthalpy h is definded as the sum of internal energy u and the flow work P / ρ:
h = u + P / ρThen the mass flow energy transfer becomes:
m(u + P/ρ + V2/2 + gz)
= m(h + V2/2 + gz)General Forms of First Law
| ∆Esys = Ein – Eout | Initial state and final state process |
| E∙sys = E∙in – E∙out | General rate process |
where:
Esys = msys(u + V2/2 + gz)sys
Ein = Qin + Win + min(h + V2/2 + gz)in
Eout = Qout + Wout + mout(h + V2/2 + gz)out
h = u + P / ρWhen we solve thermodynamic problems, we use the first and second laws and property relations to provide relationships between processes and states. In general a thermodynamic analysis means finding the values of all the processes and states involved in the problem at hand. We can only do so if the number of unknowns ≤ the number of relationships we have. For example: system kinetic and potential energy are determined from the system speed v and position x. Work and heat transfer are processes we must measure or calculate.
Equilibrium and Thermodynamic Properties
Equilibrium is important, if without it the property relations would be considerably more complicated and involve dynamic quantities. Recall we write property relations in a functional form like u = u(T, p). Properties are only a function of other properties, not any dynamic information (for example: du/dt).
Gibb’s Phase Rule
The number of properties required to specify the state of a simple compressible substance is 2: temperature T and pressure p. Gibb’s phase rule determines the number of independent properties required to specify the state (the degree of freedom).
f = 2 - φ + c
where c is the number of components, and ϕ the number of stable phases. The number 2 refers to the state variables temperature T and pressure p.
Equilibrium
Consider a closed system with fixed energy, mass and volume initially in a non-equilibrium state. If one waits long enough the measurable properties such as pressure, temperature and density will be uniform throughout and unvarying with time.
Dynamical non-equilibrium behavior is required to reach equilibrium. Formally, equilibrium is a statistical average of statistically stationary microscopic behavior. In statistics, stationarity refers to the case where the average properties (like mean, standard deviation, etc) are unchanging.
Thus thermodynamic equilibrium means that although there is a tremendous amount of action at the microscopic level, we only see unchanging average behavior at the macroscopic level.
Flow, heat transfer, mass transfer and chemical kinetics are non-equilibrium processes that change the thermodynamic state.
My Certificate
For more on Thermodynamics: Fundamental Concepts, please refer to the wonderful course here https://www.coursera.org/learn/macroscopic-microscopic-thermodynamics
Related Quick Recap
I am Kesler Zhu, thank you for visiting my website. Check out more course reviews at https://KZHU.ai